by Giulia Mantovani
In the 17th century when alchemy was at its height, many scientists were convinced that they could use science to turn more ordinary substances into gold. It was a time of transformation and potential discoveries. Even though the alchemists failed in their quest, I have become an adamant advocate of a different form of alchemy: using artistic expression to transform abstract scientific concepts into a visual medium. And I work specifically with nanoparticles, which are too small to see, so that has to count for something!

Drawn on an iPad Pro on Procreate and Canva. (Image by Giulia Mantovani, courtesy of Creative Commons CC-BY-NC-ND).
My artistic journey so far
I have been drawing since a very young age, typically depicting the real or fictional worlds around me, like scenes from Phantom of the Opera or characters from comic books. And once I got into science, I never left my need for creative expression behind, though for a while my artistic pursuits were separate from my science. In middle school I was told not to print my art on shirts for the annual holiday fair because no one had ever sold their art like that and teachers thought no one would purchase from me. Fortunately, I ignored them, learned how to iron on my own designs to cut costs, and was the first vendor at the fair to sell out my stock. Later I helped design a logo for that same middle school, which ended up being the mascot used on clothes and the website. In high school, I was the President of a t-shirt graphic design club and I went on to design the senior shirt, and during my undergraduate studies I was merchandise/design officer in my pre-health sorority.

Some of my first memorable exposures to how artists can illustrate scientific concepts came from a Japanese graphic novel and animated series by Akane Shimizu known as Cells at Work. The series personified elements of the human body to help explain the science behind their function, and that concept of finding different ways to explain science in a visual medium really stuck with me. The depictions of macrophages and platelets in Cells at Work came back to me as I learned about those concepts in college. Then in my junior year I entered a chemistry simile contest called the Sweeney Award in honor of the late chemistry professor Michael A Sweeney. I was initially satisfied with finding a creative method to explain a chemical concept, but instead of just entering a written simile, I included a small cartoon to further cement my point.

Art in Chemical Education
Visually creating a story has always helped me learn new and difficult material. When I struggled to learn multiplication tables as a kid, I would often assign personalities to numbers and visualize them moving on my page into the right answer. In biochemistry class I created colorful diagrams illustrating different metabolism pathways customizing them to get a better idea of where proteins and enzymes were coming and going. That year I had multiple classmates ask for my pathway diagrams, even some who took biochemistry after me. Putting pen to paper (or nowadays stylus to tablet), I have been able to understand and better remember scientific concepts than I would with words alone.
Using visual storytelling can be a matter of practicality when we’re communicating about things that are too small to see with the naked eye. How else can we understand things like two individual atoms reacting together to create new molecules? That is why in chemistry there are so many different methods of visually depicting reactions and molecules. Chemists understand that a visual medium is needed to further depict and comprehend what is going on at the molecular level.
Leaders in chemical education urge instructors to take a more hands-on, interdisciplinary approach that generates more excitement about learning abstract concepts in new and exciting parts of the field.1 For many students, science can seem hidden behind complicated terminology, and chemical reactions specifically are often invisible, making them hard to access. In a study comparing art-based learning and concept-based learning of chemistry in high school students, the art-based learning students showed better understanding of the material after creating art using chemistry and explaining the concept behind it. An important aspect to note about the study was that not only did the students learn better with interdisciplinary learning, but they enjoyed it more.2
Sharing Science with a Broader Audience
But what if the creativity of visual depictions could be taken to another level to illustrate higher level chemistry and to an even larger audience? Steve Jobs in 1994 said, “The most powerful person in the world is the storyteller. The storyteller sets the vision, values and agenda of an entire generation that is to come.”3 I believe that breathing more life into scientific concepts and approaching science education from more of a storytelling and artistic perspective can make an enormous impact. Art can help spread the concepts and joys of science to a broader community, which has inspired me to create a number of pieces illustrating my research, and in this blog post I want to share a few of the ones most focused on nanotechnology.
These pieces stem from wanting to create all different types of art while also following a path involving abundant scientific learning and research. Throughout the process of researching and developing my undergraduate research thesis, I created pieces of art that reflected what I was studying on the nanoscale. My aim was to create pieces that would use metaphor to explain concepts that occur on the nanoscale, to bring an invisible science more into the visible scale. The prevailing theme of all the pieces is “NanoParticles within Your Grasp”. There is a continuity across the pieces showing different hands involved in metaphorical connections to concepts relating to nanoparticles. The hands are either surrounding or involved in the explanation of nanoscience. I wanted to take the invisible abstract chemistry behind nanoparticles and make them look attainable. It is also a reference to how I have always enjoyed a more hands-on approach to education and being able to experience science to better understand it. These drawings are a hands-on approach to learning, similar to how one can see reactions happening in a lab or hold a molecule with a physical 3D ball and stick modeling kit that students often use during organic chemistry courses. One cannot physically hold a concept or pick up a singular nanoparticle, but I aimed to use the transformative power of illustrations and analogies to hopefully help people “grasp” the science mentally. For many of the pieces I left the hands devoid of color so anyone could imagine it was their hands gripping the abstract science of nanoparticles.
NanoParticles within Your Grasp

Treasure Trove of Shapes and Sizes: Nanoparticles can be synthesized in many different shapes and sizes, and this work shows them mixed all together in one handful[4]. The shapes and surfaces of a nanoparticle are integral when it comes to its reactivity and general properties. It is what helps make them unique. Creating and morphing nanoparticles into specific shapes changes how the particles interact with light, otherwise known as optical properties. Nanoparticle shape also affects reactivity in their solution environment; for example, high-curvature nanorods and nanostars lose electrons faster in a reaction than low-curvature nanosheets. The shape can also affect the nanomaterial’s interaction with proteins 4. Shape can be changed by carefully changing the conditions in which particles are formed. By synthesizing different shapes of the nanoparticles, researchers take advantage of all these different shape-dependent properties for research and technology. The reactivity of a nanoparticle is also affected by its shape, more specifically the flaws found in the internal geometry of a crystalline solid known as its crystallographic defects. When the crystalline structure contains a defect, it generates irregular arrangements on the crystal surface. The reactivity itself changes proportionally to the number of defects, because defects may contain incomplete bonding, which induces higher reactivity.5
Moody Influences
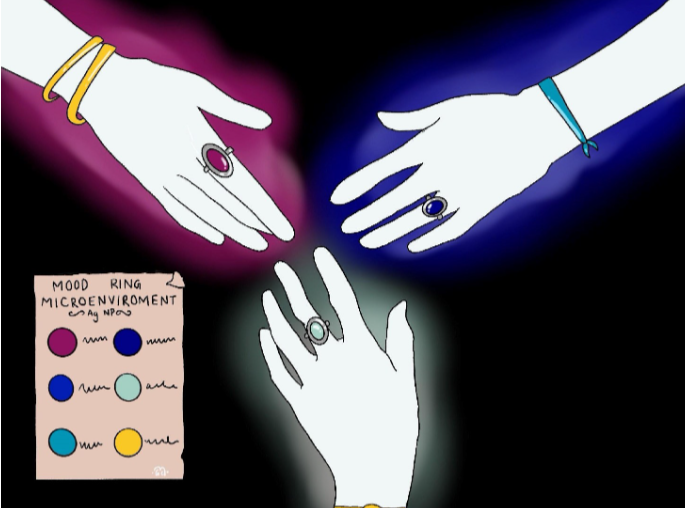
Shape isn’t the only thing about nanoparticles that can be influenced during their formation. The pH (or acidity) of the solution during the formation of gold nanorods, for example, can specifically vary the optical properties. When the nanorods are prepared in a more acidic solution, they absorb lower wavelengths of light and end up looking more red, while a more basic pH produces a shift into absorbing a higher wavelength so the solution appears more blue 6. The influence of nanoparticles by their environment can change many aspects of their properties like what kind of microenvironments are created on their surface. This reminds me of how mood rings change depending on the wearer’s “mood,” which inspired this artwork. Each person is a solution/environment creating their own unique influence on the color of the ring. (The color of mood rings is actually based on body temperature, but as a child I genuinely thought I could change the color based on my moods because my friends would have different colors than me!)
Ratios from Bitter to Sweet

This piece illustrates the varying surface-to-volume ratios for atoms of varying diameters nanoparticles using the metaphor of rims of ceramic coffee cups. One of the reasons nanoparticles are so highly reactive is that they have a high ratio of surface atoms relative to interior (non-surface) atoms. That means proportionally there are more atoms on the surface of a nanoparticle than on the interior as compared to a larger particle of the same material. The higher reactivity can lead to instability, which facilitates its ability to combine with other atoms[5]. In the case of this metaphor, the surface atoms are equivalent to the ceramic cup and the inner atoms are represented by the coffee itself. This is similar to looking at a cross sectional view of a spherical nanoparticle. The smaller espresso coffee cup has a higher ratio of rim to coffee, while the larger cappuccino cup has a higher coffee-to-rim ratio. Essentially, the different coffee sizes will have similar thickness of their cup material, but as the coffee order changes the volume of the drink changes much more than the surface of the cups. Nanoparticles are like the tiniest possible espresso cups, while “bulk” particles are more like the big cappuccino mug.
The Light Within Your Grasp

One thing you may have heard about silver nanoparticles is that they are anti-microbial – this feature is used within a variety of commercial products. Similar to gold nanoparticles, silver nanoparticles also have interesting optical properties (you can find a more in depth explanation here). These optical properties can be influenced by factors such as nanoparticle diameter and/or shape. You may notice that the colors used in the art piece don’t look like a classic rainbow; instead, I used the Localized Surface Plasmon Resonance spectrum (LSPR) from a study that was able to produce twelve colloidal silver nanoparticles across the rainbow spectrum6. The Light Within Your Grasp depicts how the varying diameters of nanoparticles can shift the wavelengths and in turn shift the color of solution produced. As the diameter of the nanoparticle increases so does the wavelength. This is one of the first unique things I learned about nanoparticles my freshman year of college and it has stuck with me ever since. It is the initial nanoparticle property that inspired it all.
Artistic inspirations
I am far from being a pioneer of the interdisciplinarity of depicting science with art. Scientists and artists have inspired each other’s works throughout history, and, in some cases, there were no clear distinctions between the two. Galileo studied art to accurately depict what he saw in his telescope; Picasso used his art to develop a scientific approach to his art, like with cubism.7 There are also many modern examples of artists turned scientists, scientists promoting art, or the two fields occurring in tandem. A few of my favorites include:
- Dr. Cathy Murphy (a CSN faculty member!), used her background as a chemist to introduce interdisciplinary science and art programs. Cathy Murphy is an American chemist who teaches at University of Illinois Urbana-Champaign. Murphy discussed multiple details about her projects in an interview published on the Sustainable Nano Podcast. Murphy explains how she started a program to have artists and scientists explain their work to each other. The STEM students got much better at explaining science concepts to well educated people who did not specialize in science.8
- Kate Nichols was the first artist in residence in the Alivisatos Lab at UC Berkeley. When beginning her work with nanoparticles, Nichols was inspired by the sensitivity of light of silver used in photography, and took the phenomenon further into the nanoscale. She leveraged subtle changes in the nanoparticles and their environment to completely transform a piece. Nichols mainly harnesses the optical properties of nanoparticles and uses them to her advantage to create unique works of art, all while using science.9
- Corrine Takara took an interdisciplinary approach from an early age as an educator, artist and engineer. Takara is based in Honolulu and identifies as an artist and STEAM educator. Much of her work involves bringing in voices and conserving heritage while also having artworks collide with science. Takara believes that her interdisciplinary work can bring in the voices of non-scientists into science. Takara has said that when looking at the role of art in science she wanted to expand science spaces and what is considered science. She talks about how science is traditionally an isolated space but it can be improved by bringing in more voices.10
As I’ve learned about nanoscience and nanotechnology, I was inspired by my own desire to create and followed the example of Kate Nichols, Dr. Cathy Murphy, and Corrine Takara. I aim to use art to interpret nanoparticle properties through visual metaphors of nanoscale phenomena. The theme of these is “Nanoparticles within Your Grasp,” using hands to either surround or explain nanoscience ideas. The captions in my drawings throughout this piece illustrate and connect these nanoscience ideas to my own life. Art clearly has an important role to play in chemistry and the greater sciences. My hope is that more educators and anyone interested in science are able to see that abstract concepts can be made “visible” with a different approach. No one should be excluded from science due to intimidation or a preconceived notion that it is not for them.
REFERENCES
- Palermo, A. (n.d.). Future of the Chemical Sciences. Retrieved from https://www.rsc.org/globalassets/04-campaigning-outreach/campaigning/future-chemicalsciences/future-of-the-chemical-science-report-royal-society-of-chemistry.pdf
- Danipog, D. L., & Ferido, M. B. (2011). Using art-based chemistry activities to improve students’ conceptual understanding in Chemistry. Journal of Chemical Education, 88(12),1610–1615. DOI: 10.1021/ed100009a
- Dykes, B. (2024). The Future Of Data Storytelling Is Augmented, Not Automated. Forbes. Retrieved from https://www.forbes.com/sites/brentdykes/2024/02/27/the-future-of-data-storytelling-is-augmented-not-automated/
- Kinnear, C., Moore, T. L., Rodriguez-Lorenzo, L., Rothen-Rutishauser, B., & Petri-Fink, A. (2017). Form follows function: Nanoparticle shape and its implications for nanomedicine. Chemical Reviews, 117(17), 11476–11521. DOI: 10.1021/acs.chemrev.7b00194
- Huang, T., & Xu, X.-H. N. (2010). Synthesis and characterization of tunable rainbow colored colloidal silver nanoparticles using single-nanoparticle plasmonic microscopy and Spectroscopy. Journal of Materials Chemistry, 20(44), 9867. DOI: 10.1039/c0jm01990a
- Smitha, S.L., et al. (2013) Size-Dependent Optical Properties of AU Nanorods. Progress in Natural Science: Materials International, 23, 1: 36–43, DOI: 10.1016/j.pnsc.2013.01.005
- Miller, A. I. (2019). The colliding worlds of Art and science. Nature Nanotechnology. 14, 400. DOI: 10.1038/s41565-019-0448-4
- Krause, M. (2016, December 22). EP 11. When artists and scientists collaborate. Sustainable Nano Podcast. Retrieved from https://sustainable-nano.com/2016/12/20/ep-11-when-artists-and-scientists-collaborate/
- Kate Nichols. (n.d.) retrieved from https://www.katenicholsstudio.com/#/looking-glass/
- Takara, C. (n.d.). Okada design. Retrieved from https://www.okadadesign.com/#/collections/